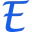Which pure metal in its solid form in oxygen has the highest ignition temperature in degrees Fahrenheit?
A . Aluminum
B . Potassium
C . Titanium
D . Zirconium
Answer: C
Explanation:
According to the web search results, titanium is the pure metal in its solid form in oxygen that has the highest ignition temperature in degrees Fahrenheit. The spontaneous-ignition temperature of titanium in the solid phase (below melting) is about 3020°F (1660°C)1. Aluminum, potassium, and zirconium have lower ignition temperatures in the solid phase, ranging from 1292°F (700°C) to 1652°F (900°C)23.
References:
IGNITION OF METALS IN HIGH PRESSURE OXYGEN2
Combustion of Metals in Oxygen-Enriched Atmospheres3
High-temperature oxidation and ignition of metals1
Latest CFPS Dumps Valid Version with 102 Q&As
Latest And Valid Q&A | Instant Download | Once Fail, Full Refund